
Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric acid. hcl(aq), as described by the chemical equationmno2(s) + 4hcl(aq) --> mncl2(aq) + 2h2o(l) + cl2(g)how much mno2(s) should be added to excess hcl (aq) to obtain 235 ml of cl2(g) at 25 degrees c and 805 torr?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 12:00
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 16:00
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1
You know the right answer?
Chlorine can be prepared in the laboratory by the reaction of manganese dioxide with hydrochloric ac...
Questions

Mathematics, 05.03.2021 18:20




Mathematics, 05.03.2021 18:20

Mathematics, 05.03.2021 18:20


Mathematics, 05.03.2021 18:20



Mathematics, 05.03.2021 18:20


Mathematics, 05.03.2021 18:20

Mathematics, 05.03.2021 18:20





Mathematics, 05.03.2021 18:20

Mathematics, 05.03.2021 18:20

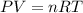
![25^oC=[25+273]K=298K](/tpl/images/0431/2060/df1f6.png)
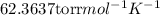

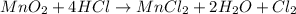
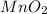 = 0.01017 moles
= 0.01017 moles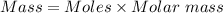
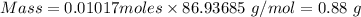
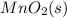 should be added to excess HCl (aq) to obtain 235 mL of
should be added to excess HCl (aq) to obtain 235 mL of 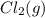 at 25 degrees C and 805 Torr.
at 25 degrees C and 805 Torr.

