
Chemistry, 24.12.2019 19:31 jeifetz1023
What is the theoretical yield of aluminum that can be produced by the reaction of 60.0 g of aluminum oxide with 30.0 g of carbon according to the following chemical equation? al2o3 + 3c → 2al + 3co

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:30
(apex) when a cup of water is dropped, as the cup falls, the water in the cup falls out true or false?
Answers: 1

Chemistry, 22.06.2019 07:30
Aradio signal from a gps satellite take only about 0.067 seconds to reach a gps reciever. if the speed of light is about 300,000km/s, then approximately how far away is the reciever from from the satellite?
Answers: 1

Chemistry, 22.06.2019 15:00
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. p k a1 p k a2 1.30 6.70 calculate the ph for each of the points in the titration of 50.0 ml of 1.5 m h3po3(aq) 1.5 m h 3 po 3 ( aq ) with 1.5 m koh(aq). 1.5 m koh ( aq ) .
Answers: 1

You know the right answer?
What is the theoretical yield of aluminum that can be produced by the reaction of 60.0 g of aluminum...
Questions











Mathematics, 14.01.2021 15:40






Mathematics, 14.01.2021 15:40


Spanish, 14.01.2021 15:40

Biology, 14.01.2021 15:40

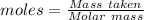
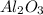
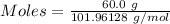
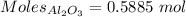
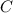
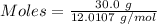
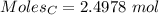
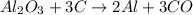
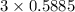 moles of carbon
moles of carbon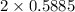 moles of aluminium.
moles of aluminium.

