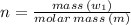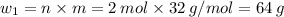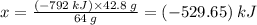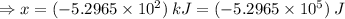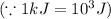Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1


Chemistry, 23.06.2019 11:50
Achemist needs to prepare a buffer solution of ph 8.80. what molarity of nh3 (pkb = 4.75) is required to produce the buffer solution if the (nh4)2so4 in the solution is 1.8 m?
Answers: 1
You know the right answer?
Sulfur undergoes combustion to yield sulfur trioxide by the following reaction equation:
Questions




Mathematics, 05.05.2020 01:01


Mathematics, 05.05.2020 01:01






Mathematics, 05.05.2020 01:01




Arts, 05.05.2020 01:01


Business, 05.05.2020 01:01


Mathematics, 05.05.2020 01:01