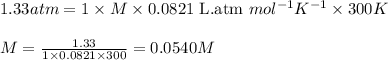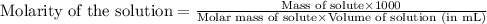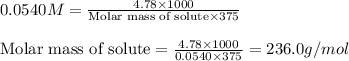Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:30
Write a paragraph that provides examples of each stage of volcanic activity, a description of the volcano, and facts about each stage.
Answers: 1

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3
You know the right answer?
Asolution is prepared by dissolving 4.78 g of an unknown nonelectrolyte in enough water to make 375...
Questions

Mathematics, 12.05.2021 17:20

Mathematics, 12.05.2021 17:20


Mathematics, 12.05.2021 17:20

Geography, 12.05.2021 17:20




Mathematics, 12.05.2021 17:20





Mathematics, 12.05.2021 17:20

Mathematics, 12.05.2021 17:20


Mathematics, 12.05.2021 17:20


Chemistry, 12.05.2021 17:20

Arts, 12.05.2021 17:20

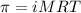
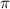 = osmotic pressure of the solution = 1.33 atm
= osmotic pressure of the solution = 1.33 atm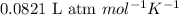
![27^oC=[273+27]K=300K](/tpl/images/0434/0272/00f96.png)