

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1


Chemistry, 22.06.2019 21:50
Answer the questions about this reaction: nai(aq) + cl2(g) → nacl(aq) + i2(g) write the oxidation and reduction half-reactions: oxidation half-reaction: reduction half-reaction: based on the table of relative strengths of oxidizing and reducing agents (b-18), would these reactants form these products? write the balanced equation: answer options: a. 0/na -> +1/na+1e- b. nai(aq) + cl2(g) → nacl(aq) + i2(g) c. +1/na+1e- -> 0 /na d. -1/2i -> 0/i2+2e- e. no f. 4nai(aq) + cl2(g) → 4nacl(aq) + i2(g) g. 2nai(aq) + cl2(g) → 2nacl(aq) + i2(g) h. 4nai(aq) + 2cl2(g) → 4nacl(aq) + 2i2(g) i. nai(aq) + cl2(g) → nacl(aq) + i2(g) j. 0/cl2+2e -> -1/2cl- k. yes
Answers: 1

You know the right answer?
For the reaction, 2cr2+ + cl2(g) > 2cr3+ + 2cl- e cell (standard conditions) = 1.78v calculate e...
Questions

Mathematics, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00


Mathematics, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00

History, 13.03.2021 01:00



Mathematics, 13.03.2021 01:00


Mathematics, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00



Chemistry, 13.03.2021 01:00

Mathematics, 13.03.2021 01:00

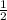 . It doesn't influence the value of the cell potential.
. It doesn't influence the value of the cell potential.

