
Chemistry, 28.12.2019 00:31 quincyjosiah07
How much heat is absorbed/released when 20.00 g of nh3(g) reacts in the presence of excess o2 (g) to produce no (g) and h2o (l) according to the following chemical equation? 4nh3 (g) + 5o2 (g) > 4no (g) +6h2o (l) δ h: +1168 kja. 342.9 kj of heat are absorbed. b. 342.9 kj of heat are released. c. 1372 kj of heat are absorbed. d. 1372 kj of heat are released.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:40
Which of the following might a chemist choose to study? a. glacier movement in alaska b. better ways to recycle plastics c. the effects of hurricanes on turtle populations d. the vibrations in bridges caused by big trucks
Answers: 2


Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 09:30
Right anwser gets marked brainliest newton's discovery concerning how fast an object will change speed is the: 1st law 2nd law 3rd law universal gravitation
Answers: 1
You know the right answer?
How much heat is absorbed/released when 20.00 g of nh3(g) reacts in the presence of excess o2 (g) to...
Questions

Mathematics, 03.03.2021 21:20



Mathematics, 03.03.2021 21:20

Mathematics, 03.03.2021 21:20


Chemistry, 03.03.2021 21:20

Mathematics, 03.03.2021 21:20

English, 03.03.2021 21:20



English, 03.03.2021 21:20

Mathematics, 03.03.2021 21:20

Biology, 03.03.2021 21:20





History, 03.03.2021 21:20

Mathematics, 03.03.2021 21:20

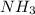 as:-
as:-
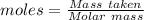
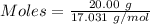
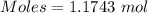
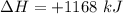
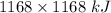 of heat
of heat

