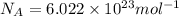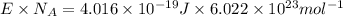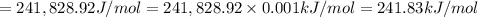Chemistry, 03.01.2020 20:31 abraralzaher
The longest wavelength of light with enough energy to break the cl-cl bond in cl2(g) is 495 nm.
1) calculate the frequency in s-1 of the light2) calculate the energy in j of a photon of the light3) calculate the minimun energy in kj mol-1 of the cl-cl bond

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 17:30
I'm learning about the periodic tables and what each subject's configuration is. for example, hydrogen is 1s^1, but i don't understand how you get that. can someone me understand how to figure out how to figure this out? sorry if the question makes no sense, but it would really a lot if you could me understand! you so much if you can!
Answers: 1


Chemistry, 23.06.2019 00:00
What conclusion can you draw from this experiment about the components of the black ink?
Answers: 3
You know the right answer?
The longest wavelength of light with enough energy to break the cl-cl bond in cl2(g) is 495 nm.
Questions









Computers and Technology, 29.07.2019 22:20

Chemistry, 29.07.2019 22:20

Mathematics, 29.07.2019 22:20



Mathematics, 29.07.2019 22:20


Mathematics, 29.07.2019 22:20


History, 29.07.2019 22:20


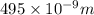
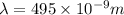
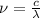
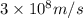
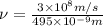
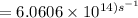
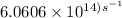 .
.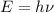
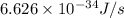
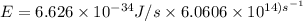

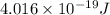 .
.