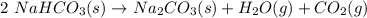Chemistry, 14.01.2020 06:31 webbskyler
Nahco3(s) --> na2co3 (s) + h20 (g) + co2 (g)
problem #1:
in order to proceed through this task - you need to balance the equation of the decomposition of baking
soda.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 03:50
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 23:00
Movement that is like a t a type of wave that transfers energy where the particles in the medium move in a circle motion while the energy travels left or right. a type of wave that transfers energy where the particles in the medium move perpendicular to the direction in which the energy is traveling. transfers energy from one location to another a type of wave that transfers energy where the particles in the medium move parallel to the direction in which the energy is traveling. movement that is back and forth, like an equal sign = 1. wave 2. parallel movement 3. perpendicular movement 4. transverse wave 5. longitudinal wave 6. surface wave
Answers: 1

Chemistry, 23.06.2019 03:30
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1

Chemistry, 23.06.2019 07:00
Why do the strengths of london (dispersion) forces generally increase with increasing molecular size? choose one: a. heavier atoms have stronger attractions for each other than lighter atoms. b. dispersion forces are all equal in magnitude; there is no size dependence. c. dispersion forces arise from the attraction between the nuclei of atoms, and larger molecules have larger nuclei. d. dispersion forces arise from dipoles caused by the electron distribution being distorted. larger molecules have more electrons and, therefore, more distortions and a bigger force. e. dispersion forces depend on distance. larger molecules are farther apart and so the forces are smaller.
Answers: 2
You know the right answer?
Nahco3(s) --> na2co3 (s) + h20 (g) + co2 (g)
problem #1:
in order to proceed throug...
problem #1:
in order to proceed throug...
Questions







English, 29.06.2019 15:30



Biology, 29.06.2019 15:30

Mathematics, 29.06.2019 15:30




History, 29.06.2019 15:30

Chemistry, 29.06.2019 15:30

Geography, 29.06.2019 15:30


Mathematics, 29.06.2019 15:30