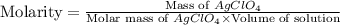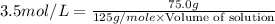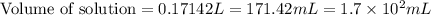Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which of the following mining methods disrupts the sea floor?
Answers: 1

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 23.06.2019 01:00
Which of the following is in the lanthanide family? a) uranium b) promethium c) silver d) gold
Answers: 2
You know the right answer?
Calculate the volume in milliliters of a 3.5 mp; /l silver perchlorate solution that contains 75.0 g...
Questions


English, 03.02.2021 01:00

Mathematics, 03.02.2021 01:00

Mathematics, 03.02.2021 01:00

Mathematics, 03.02.2021 01:00

Mathematics, 03.02.2021 01:00

Physics, 03.02.2021 01:00





Mathematics, 03.02.2021 01:00

Mathematics, 03.02.2021 01:00


Mathematics, 03.02.2021 01:00

Computers and Technology, 03.02.2021 01:00

Mathematics, 03.02.2021 01:00



Mathematics, 03.02.2021 01:00

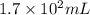
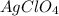 = 75.0 g
= 75.0 g