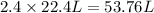Chemistry, 16.01.2020 22:31 BreBreDoeCCx
When calcium carbonate is added to hydrochloric acid, calcium chloride, carbon dioxide, and water are produced according to the balanced chemical equation below:
caco3(s) + 2hc1(aq) 4 cac12(aq) +h20(1) + co2(g)
imagine mixing 2.5 moles of calcium carbonate with 4.8 moles of hydrochloric acid. calculate the number of moles of caco3 and hcl used in the intro activity. determine the limiting reactant and use the ideal gas law to estimate the max volume of co2

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 11:00
The twister and runaway train are two coasters at the same amusement park. both coasters start at the same height. the coaster for the twister is twice the mass of the coaster for the runaway train. which roller coaster has greater gravitational potential energy at the start of the ride?
Answers: 1

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

You know the right answer?
When calcium carbonate is added to hydrochloric acid, calcium chloride, carbon dioxide, and water ar...
Questions

Arts, 23.06.2021 05:30



English, 23.06.2021 05:30


English, 23.06.2021 05:30


History, 23.06.2021 05:30

Spanish, 23.06.2021 05:30

Mathematics, 23.06.2021 05:30

English, 23.06.2021 05:30

Mathematics, 23.06.2021 05:30

Mathematics, 23.06.2021 05:30




Mathematics, 23.06.2021 05:30

Mathematics, 23.06.2021 05:30

Social Studies, 23.06.2021 05:30

Mathematics, 23.06.2021 05:30

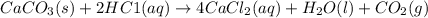
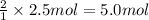 of HCl
of HCl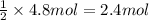 of calcium carbonate
of calcium carbonate