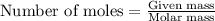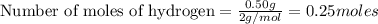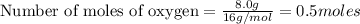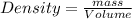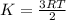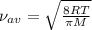Chemistry, 18.01.2020 01:31 josiebranstetter93
Two flexible containers for gases are at the same temperature and pressure. one holds 0.50 gram of hydrogen and the other holds 8.0 grams of oxygen. which of the following statements regarding these gas samples is false? the volume of the hydrogen container is the same as the volume of the oxygen container.
a the number of molecules in the hydrogen container is the same as the number of molecules in the oxygen container.
b the density of the hydrogen sample is less than that of the oxygen sample.
c the average kinetic energy of the hydrogen molecules is the same as the average kinetic energy of the oxygen molecules.
d the average speed of the hydrogen molecules is the same as the average speed of the oxygen molecules.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3
You know the right answer?
Two flexible containers for gases are at the same temperature and pressure. one holds 0.50 gram of...
Questions


Spanish, 04.12.2021 01:10

Mathematics, 04.12.2021 01:10







Mathematics, 04.12.2021 01:10




Mathematics, 04.12.2021 01:10






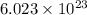 of particles.
of particles.