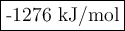()
the equation for the reaction can be shown as:
hh
h-c-0-0-h
+ 30=
...

Chemistry, 19.01.2020 17:31 brooklyn4682
()
the equation for the reaction can be shown as:
hh
h-c-0-0-h
+ 30=
0
2
0=c=o
+ 3h-o-h
hh
bond
bond energy in
kj per mole
413
347
c-h
c-c
c-o
c=0
o-h
o=0
358
799
467
495
use the bond energies to calculate the overall energy change for this
reaction.
overall energy change =


Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 19:30
The molecular formula for caffeine is cshion402. which of the following elements is not found in caffeine?
Answers: 1

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 22.06.2019 13:00
These questions are based on the attached photo. the experiment is about burning magnesium metal with oxygen. 1. write the balanced chemical equation for the reaction you are performing. 2. calculate the mass of magnesium metal used in each trial. o trial 1: o trial 2: 3. calculate the actual yield of magnesium oxide for each trial. o trial 1: o trial 2: 4. magnesium is the limiting reactant in this experiment. calculate the theoretical yield of mgo for each trial. o trial 1: o trial 2: 5. determine the percent yield of mgo for your experiment for each trial. o trial 1: o trial 2: 6. determine the average percent yield of mgo for the two trials. your company currently uses a process with a similar cost of materials that has an average percent yield of 91 percent. if the average percent yield of this process is higher than that, this could save the company money. what is your recommendation to the company? support your recommendation using your data, calculations, and understanding of stoichiometry gathered from this lab.
Answers: 1

Chemistry, 22.06.2019 14:30
Select all that apply. using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 (s) pb+2(aq) + 2cl -(aq). the concentration of the products yield a ksp of 2.1 x 10-2:
Answers: 2
You know the right answer?
Questions













History, 28.07.2019 06:00


English, 28.07.2019 06:00



History, 28.07.2019 06:00


History, 28.07.2019 06:00