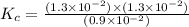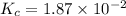Chemistry, 25.12.2019 00:31 valenciafaithtorres
Consider the following reaction: . so2cl2(g)⇌so2(g)+cl2(g) . a reaction mixture is made containing an initial [so2cl2] of 2.2×10−2m . at equilibrium, [cl2]= 1.3×10−2m .. calculate the value of the equilibrium constant (kc).

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:00
How is the composition of a meteorite relevant to finding out the composition of earth's core?
Answers: 3

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2
You know the right answer?
Consider the following reaction: . so2cl2(g)⇌so2(g)+cl2(g) . a reaction mixture is made containing...
Questions


Physics, 20.07.2019 14:10

Chemistry, 20.07.2019 14:10

Health, 20.07.2019 14:10












Business, 20.07.2019 14:10




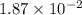
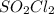 =
= 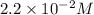
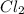 =
= 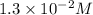

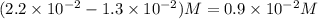
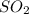 =
= ![K_c=\frac{[SO_2]\times [Cl_2]}{[SO_2Cl_2]}](/tpl/images/0432/3201/33429.png)