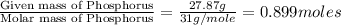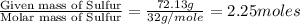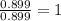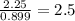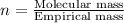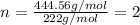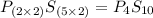Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1

Chemistry, 22.06.2019 12:20
Which is an example of the practical pursuit of alchemy? a. forming perfect substances. b. transforming base metals. c. developing metalworking techniques. d. linking spiritual characteristics with material substances.
Answers: 1
You know the right answer?
Ferrophosphorus (fe2p) reacts with pyrite (fes2), producing iron (ii) sulfide and a compound that is...
Questions

Medicine, 15.04.2020 15:33



Physics, 15.04.2020 15:33


Mathematics, 15.04.2020 15:33





Mathematics, 15.04.2020 15:33





History, 15.04.2020 15:33

Biology, 15.04.2020 15:33



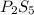 and
and 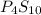 respectively.
respectively.