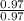Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which term best describes the form sound takes as it travels away from a drum (a- gas)(b-music) ( c-waves) (d-particles
Answers: 3

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 23.06.2019 01:00
The time that is taken by neptune once around the sun is called
Answers: 1

Chemistry, 23.06.2019 04:10
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2
You know the right answer?
An unknown compound contains 75.69% carbon, 8.80% hydrogen, and 15.51% oxygen by mass. a mass spectr...
Questions


Mathematics, 18.03.2021 18:10

Health, 18.03.2021 18:10






World Languages, 18.03.2021 18:10

Biology, 18.03.2021 18:10

Mathematics, 18.03.2021 18:10


Mathematics, 18.03.2021 18:10


History, 18.03.2021 18:10

History, 18.03.2021 18:10



Mathematics, 18.03.2021 18:10

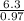 H -
H -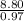 O -
O -