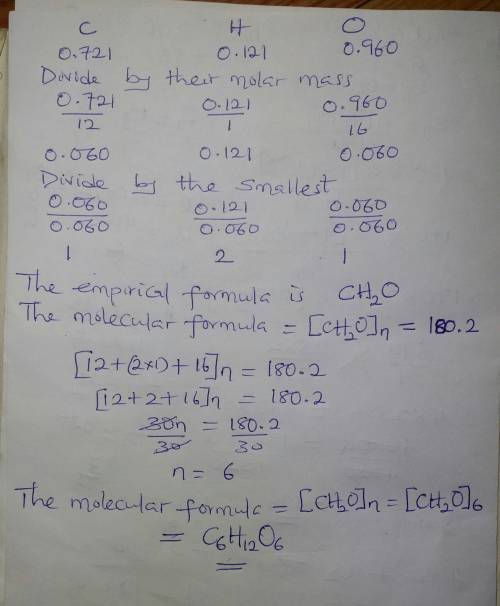Chemistry, 11.02.2020 00:34 bikerhomie
4. A 1.802 gram sample of unknown compound contains 0.721 grams of carbon, 0.121 grams of hydrogen and 0.960 grams of oxygen. The compound has a molar mass of 180.2 g/mol. Determine the empirical formula of the compound and the molecular formula.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 13:30
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1
You know the right answer?
4. A 1.802 gram sample of unknown compound contains 0.721 grams of carbon, 0.121 grams of hydrogen a...
Questions




Mathematics, 17.10.2020 23:01



Mathematics, 17.10.2020 23:01


Business, 17.10.2020 23:01

History, 17.10.2020 23:01


Business, 17.10.2020 23:01


Social Studies, 17.10.2020 23:01


Mathematics, 17.10.2020 23:01


History, 17.10.2020 23:01

Mathematics, 17.10.2020 23:01

Social Studies, 17.10.2020 23:01