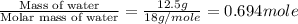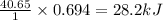Answers: 1
Another question on Chemistry

Chemistry, 23.06.2019 10:30
When a chemist collects hydrogen gas over water, she ends up with a mixture of hydrogen and water vapor in her collecting bottle. if the pressure in the collecting bottle is 97.1 kilopascals and the vapor pressure of the water is 3.2 kilopascals, what is the partial pressure of the hydrogen?
Answers: 1

Chemistry, 23.06.2019 16:30
In chile, the deepest earthquake occurred at 61.7°w longitude at a depth of 540 km. if the rocks at the focus began subducting 10 million years ago and are now 1000 km from their original position, what is the average rate of subduction in cm/yr?
Answers: 1

Chemistry, 23.06.2019 21:30
Which particles make up the nucleus of an atom? a. protons and electrons b. neutrons and electrons c. protons only d. protons and neutrons e. neutrons only
Answers: 2

Chemistry, 23.06.2019 21:50
Agas engine that operates on a brayton cycle has an efficiency of 0.23. on a cold day, the temperature of the air drawn into the engine is 267 k.part awhat is the temperature of the air exhausted from the engine?
Answers: 3
You know the right answer?
The enthalpy of vaporization of liquid water is 40.65 kJ/mol. Calculate the energy required to vapor...
Questions

Mathematics, 24.11.2020 16:40

English, 24.11.2020 16:40














Mathematics, 24.11.2020 16:40



English, 24.11.2020 16:40

Mathematics, 24.11.2020 16:40