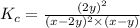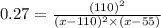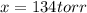Nitric oxide reacts with chlorine gas according to the following reaction:
2NO(g)+Cl2(g)?2NOC...

Chemistry, 12.02.2020 02:45 ghaithalhamdani
Nitric oxide reacts with chlorine gas according to the following reaction:
2NO(g)+Cl2(g)?2NOCl(g)
Kp=0.27 at 700 K
A reaction mixture initially contains equal partial pressures of NO and Cl2. At equilibrium, the partial pressure of NOCl was measured to be 110 torr. What were the initial partial pressures of NO and Cl2?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 23:00
What is a substance? a. a physical property of matter b. a chemical property of matter c. an element or compound that cannot be physically separated d. characteristics used to tell the difference between mixtures
Answers: 1

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1

Chemistry, 23.06.2019 07:00
What are the trends and exceptions to the trends in electron affinity?
Answers: 1
You know the right answer?
Questions


Mathematics, 22.07.2019 13:00

Computers and Technology, 22.07.2019 13:00

Mathematics, 22.07.2019 13:00

Mathematics, 22.07.2019 13:00







Chemistry, 22.07.2019 13:00

Mathematics, 22.07.2019 13:00

Biology, 22.07.2019 13:00

Mathematics, 22.07.2019 13:00


Mathematics, 22.07.2019 13:00

Mathematics, 22.07.2019 13:00


English, 22.07.2019 13:00

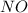 and
and 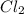 are 134 torr each.
are 134 torr each.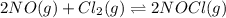
![K_c=\frac{[NOCl]^2}{[NO]^2[Cl_2]}](/tpl/images/0507/8534/56950.png)