
Chemistry, 12.02.2020 05:28 friendsalwaysbae
For a ternary solution at constant T and P, the composition dependence of molar property M is given by: M = x1M1 + x2M2 + x3M3 + x1 x2 x3C where M1, M2, and M3 are the values of M for pure species 1, 2, and 3, and C is a parameter independent of composition. Determine expressions for M¯1,M¯2, and M¯3 by application of Eq. (10.7). As a partial check on your results, verify that they satisfy the summability relation, Eq. (10.11). For this correlating equation, what are the M¯i at infinite dilution?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 06:10
Explain the relationship between forward and backward reactions in equilibrium, and predict how changing the amount of a reactant (creating a tension) will affect that relationship.
Answers: 1

Chemistry, 22.06.2019 06:20
If i can still dissolve more sugar into the solution at a certain temperature what would i call that solution
Answers: 3

Chemistry, 22.06.2019 21:20
One way in which the useful metal copper is produced is by dissolving the mineral azurite, which contains copper(ii) carbonate, in concentrated sulfuric acid. the sulfuric acid reacts with the copper(ii) carbonate to produce a blue solution of copper(ii) sulfate. scrap iron is then added to this solution, and pure copper metal precipitates out because of the following chemical reaction: (s) (aq) (s) (aq) suppose an industrial quality-control chemist analyzes a sample from a copper processing plant in the following way. he adds powdered iron to a copper(ii) sulfate sample from the plant until no more copper will precipitate. he then washes, dries, and weighs the precipitate, and finds that it has a mass of .
Answers: 2
You know the right answer?
For a ternary solution at constant T and P, the composition dependence of molar property M is given...
Questions





Health, 03.04.2020 22:30

Mathematics, 03.04.2020 22:30

Mathematics, 03.04.2020 22:30





English, 03.04.2020 22:30





Mathematics, 03.04.2020 22:30



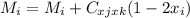 ...1
...1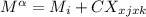 ...2
...2![M_{i} = [\frac{d(nM)}{dn_{i} }]_{P,t,n,j}](/tpl/images/0508/2556/4b4ae.png)


