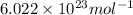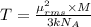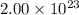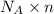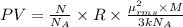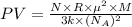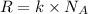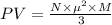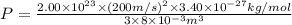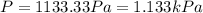Chemistry, 17.02.2020 23:47 YannahRussell
A cubic box with sides of 20.0 cm contains 2.00 × 1023 molecules of helium with a root-mean-square speed (thermal speed) of 200 m/s. The mass of a helium molecule is 3.40 × 10-27 kg. What is the average pressure exerted by the molecules on the walls of the container? (The Boltzmann constant is 1.38 × 10-23 J/K and the ideal gas constant is R = 8.314 J/mol•K .) (12 pts.)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2


Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
A cubic box with sides of 20.0 cm contains 2.00 × 1023 molecules of helium with a root-mean-square s...
Questions



Mathematics, 25.01.2021 16:50



Mathematics, 25.01.2021 16:50

Biology, 25.01.2021 16:50

Mathematics, 25.01.2021 16:50

English, 25.01.2021 16:50



Mathematics, 25.01.2021 16:50

Mathematics, 25.01.2021 16:50


Mathematics, 25.01.2021 16:50


Mathematics, 25.01.2021 16:50



Mathematics, 25.01.2021 16:50

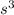
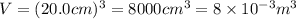
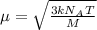
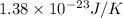
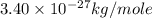
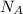 = Avogadro’s number =
= Avogadro’s number =