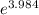The activation energy for the isomerization ol cyclopropane to propene is 274 kJ/mol. By what factor does the rate of this reaction increase as the temperature rises from 255 to 291 oC? Hint: The factor is the ratio of the rates. Since rate is directly proportional to rate constant, the factor is also ratio of rate constants.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 12:30
If anyone would be able to me out with these three questions it would be these are from the chem 2202 course.
Answers: 3

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
You know the right answer?
The activation energy for the isomerization ol cyclopropane to propene is 274 kJ/mol. By what factor...
Questions



Mathematics, 29.01.2020 11:50

Geography, 29.01.2020 11:50




Computers and Technology, 29.01.2020 11:50


History, 29.01.2020 11:50

Advanced Placement (AP), 29.01.2020 11:50

Biology, 29.01.2020 11:50


Chemistry, 29.01.2020 11:50




Advanced Placement (AP), 29.01.2020 11:50

Mathematics, 29.01.2020 11:50

English, 29.01.2020 11:50

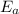 ) = 274 kJ/mol.
) = 274 kJ/mol. 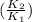 =
= ![\frac{E_a}{R} [\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0514/4438/3b4c3.png)
![\frac{273kJ/mol}{8.314*10^{-3}kJK^{-1}} [\frac{1}{528}-\frac{1}{564}]](/tpl/images/0514/4438/e9d63.png)
![\frac{273kJ/mol}{8.314*10^{-3}kJK^{-1}} [\frac{36k}{(528K)(564K)}]](/tpl/images/0514/4438/2ab49.png)