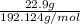Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 23.06.2019 10:30
Using the periodic table, complete the following. element: hydrogen symbol: h₂ molecular weight: g mass of one mole: g/mol
Answers: 3


Chemistry, 23.06.2019 14:00
If the molar mass of the compound is 96.69 g/mol, what is the molecular formula of the compound?
Answers: 1
You know the right answer?
A sample of citric acid, C6H8O7, has a mass of 22.9 g. What amount, in moles, does this mass represe...
Questions