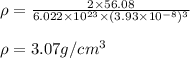Chemistry, 20.02.2020 23:03 NicoleParker
An imaginary element with BCC structure and has an atomic radius of 0.17 nm, with a molar mass of 56.08 g/mol. What is the density of this element in g/cc? hint: you will need Avogadro's number and you will need to convert the given radius to cm.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1


Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 07:10
Remember to use the proper number of significant figures and leading zeros in all calculations.gelatin has a density of 1.27 g/cm³. if you have a blob of gelatin dessert that fills a 2.0 liter bottle, what is its mass? 2540 g2500 g3.9 x 10-43.937x 10-4
Answers: 3
You know the right answer?
An imaginary element with BCC structure and has an atomic radius of 0.17 nm, with a molar mass of 56...
Questions


Mathematics, 31.03.2021 01:30

Engineering, 31.03.2021 01:30

Mathematics, 31.03.2021 01:30

Mathematics, 31.03.2021 01:30

Physics, 31.03.2021 01:30



Mathematics, 31.03.2021 01:30



Chemistry, 31.03.2021 01:30






Social Studies, 31.03.2021 01:30

Mathematics, 31.03.2021 01:30

Mathematics, 31.03.2021 01:30

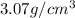
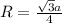
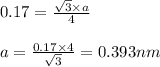
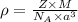
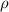 = density
= density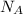 = Avogadro's number =
= Avogadro's number = 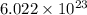
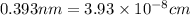 (Conversion factor:
(Conversion factor: 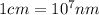 )
)