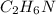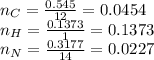Chemistry, 21.02.2020 01:05 jaceraulerson7249
The rotten smell of a decaying animal carcass is partially due to a nitrogen-containing compound called putrescine. Elemental analysis of putrescine showed that it consisted of 54.50% X, 13.73% H, and 31.77% N. find empirical formula

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1

Chemistry, 23.06.2019 13:30
The activation energy for a(n) is quite large and usually takes extra energy from the environment, it is normally not a natural spontaneous process. combustion reaction endothermic reaction exothermic reaction catalyzed reaction
Answers: 1
You know the right answer?
The rotten smell of a decaying animal carcass is partially due to a nitrogen-containing compound cal...
Questions

History, 20.04.2020 01:44


Computers and Technology, 20.04.2020 01:45


Mathematics, 20.04.2020 01:45

Mathematics, 20.04.2020 01:45



History, 20.04.2020 01:45

Biology, 20.04.2020 01:45

Computers and Technology, 20.04.2020 01:45


Mathematics, 20.04.2020 01:46


Mathematics, 20.04.2020 01:46

Biology, 20.04.2020 01:46

Arts, 20.04.2020 01:46

Mathematics, 20.04.2020 01:46