Chemistry, 21.02.2020 02:37 tishaadams2160
When iron rusts, solid iron reacts with gaseous oxygen to form solid iron(III) oxide. Enter a balanced chemical equation for this reaction. Express your answer as a chemical equation. Identify all of the phases in your answer.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 09:40
Which diagram shows the correct way to represent an ionic compound of magnesium oxide?
Answers: 3

Chemistry, 22.06.2019 19:00
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3

Chemistry, 23.06.2019 00:20
Which diagram represents the phase tha occurs after a solid melts?
Answers: 1
You know the right answer?
When iron rusts, solid iron reacts with gaseous oxygen to form solid iron(III) oxide. Enter a balanc...
Questions


Mathematics, 11.10.2021 17:20


English, 11.10.2021 17:20

Mathematics, 11.10.2021 17:20

Mathematics, 11.10.2021 17:20



Mathematics, 11.10.2021 17:20

English, 11.10.2021 17:20

Mathematics, 11.10.2021 17:20



Mathematics, 11.10.2021 17:20

Mathematics, 11.10.2021 17:20

Mathematics, 11.10.2021 17:20

Mathematics, 11.10.2021 17:20

English, 11.10.2021 17:20


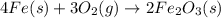
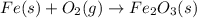
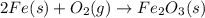
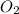 and 2 in front of
and 2 in front of 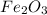 to balcne the oxygen atom.
to balcne the oxygen atom.