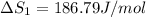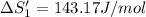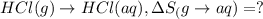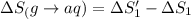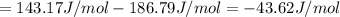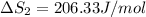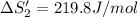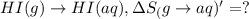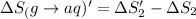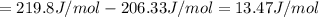All of the hydrogen halides (H-X) are gaseous in their natural state, but dissolve in water to form the aqueous phase.
Entropies (S) for the gaseous H-X molecules (before reaction) are:
HCl (g): 186.79 J/mol
HI (g): 206.33 J/mol
Entropies (S) for the H-X molecules dissolved/solvated in water (after reaction) are:
HCl (aq): 143.17 J/mol
HI (aq): 219.8 J/mol
1. Calculate the ΔS that each of these H-X compounds undergoes as it transitions from the gas phase to the aqueous phase.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 18:10
The enthalpy of formation of water is -285.8 kj/mol. what can be inferred from this statement?
Answers: 1

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3
You know the right answer?
All of the hydrogen halides (H-X) are gaseous in their natural state, but dissolve in water to form...
Questions




Health, 14.07.2019 18:00



Health, 14.07.2019 18:00



Mathematics, 14.07.2019 18:00



Mathematics, 14.07.2019 18:00

Mathematics, 14.07.2019 18:00



Mathematics, 14.07.2019 18:00


Mathematics, 14.07.2019 18:00

![\Delta S=[\text{Sum of entropy of products}]-[\text{Sum of entropy of reactants}]](/tpl/images/0519/3497/4a681.png)