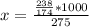Potassium hydrogen phosphate is used in the preparation of non-dairy powdered creamers. Calculate the molarity of a solution prepared by dissolving 238 g of K2HPO4 in enough water to produce 275 mL of solution. A) 0.732 M B) 0.865 M. C) 2.66 M D) 4.97 M E) none of the above

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
The first element on the periodic table of elements is carbon. a. true b. false
Answers: 2

Chemistry, 22.06.2019 15:00
Why does a plastic bottle that is sealed at a high altitude change it’s shape when taken to lower altitude
Answers: 2

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
You know the right answer?
Potassium hydrogen phosphate is used in the preparation of non-dairy powdered creamers. Calculate th...
Questions

Mathematics, 27.11.2020 02:00

Computers and Technology, 27.11.2020 02:00


Computers and Technology, 27.11.2020 02:00



Computers and Technology, 27.11.2020 02:00



Computers and Technology, 27.11.2020 02:00



Computers and Technology, 27.11.2020 02:10


Social Studies, 27.11.2020 02:10

English, 27.11.2020 02:10

Social Studies, 27.11.2020 02:10



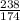 moles.
moles.