
Chemistry, 22.02.2020 03:32 bibigardeax
Mastering chem If the experiment below is run for 60 s, 0.16 mol A remain. Which of the following statements is or are true? At time 0 seconds, a flask contains 1.00 mole of A and 0 moles of B. At 20 seconds, the flask holds 0.54 moles of A and 0.46 moles of B. At 40 seconds, the flask holds 0.3 moles of A and 0.7 moles of B. After 60 s there are 0.84 mol B in the flask. The decrease in the number of moles of A from t1 = 0 to t2 = 20 s is greater than that for t1 = 40 to t2 = 60 s. The average rate for the reaction from t1 = 40 to t2 = 60 s is 7.0×10−3 M/s.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 21.06.2019 23:30
Problem #3 (ch. 1, problem 15)the ideal gas law provides one way to estimate the pressure exerted by a gas on a container. the law isí‘ťí‘ť=푛푛푛푛푛푛푉푉more accurate estimates can be made with the van der waals equationí‘ťí‘ť=푛푛푛푛푛푛푉푉â’푛푛푟푟â’푞푞푛푛2푉푉2where the term nb is a correction for the volume of the molecules and the term an2/v2is a correction for molecular attractions. the values of a and b depend on the type of gas. the gas constant is r, the absolutetemperature is t, the gas volume is v, and the number of moles of gas molecules is indicated by n. if n = 1 mol of an ideal gas were confined to a volume of v = 22.41 l at a temperature of 0â°c (273.2k), it would exert a pressure of 1 atm. in these units, r = 0.0826.for chlorine gas (cl2), a = 6.49 and b = 0.0562. compare the pressure estimates given by the ideal gas law and the van der waals equation for 1 mol of cl2 in 22.41 l at 273.2 k. what is the main cause of the difference in the two pressure estimates, the molecular volume or the molecular attractions?
Answers: 1

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3
You know the right answer?
Mastering chem If the experiment below is run for 60 s, 0.16 mol A remain. Which of the following st...
Questions






Mathematics, 10.04.2020 03:20






Biology, 10.04.2020 03:20

Mathematics, 10.04.2020 03:20



Mathematics, 10.04.2020 03:20



Mathematics, 10.04.2020 03:20

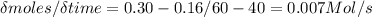 which is true as well
which is true as well 

