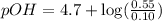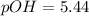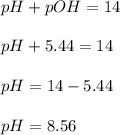Chemistry, 25.02.2020 02:57 fangirl2837
What is the pH of a solution that results when 0.010mol HNO3 is added to 500.ml of a solution that is 0.10M in aqueous ammonia and 0.55 M in ammonium nitrate. assume no volume change. (The Kb for NH3 =1.8 * 10-5 )

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 23:00
The wave shown on the electromagnetic spectrum disturb the medium it passes through a)different frequency. b)the same frequency .
Answers: 2

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 15:20
Select the most likely product for this reaction: koh(aq) + co2(g) – ? k2co3(aq) + h2o(1) k(s) + h2(g) + o2(g) k(s) + co3(9) +h2
Answers: 2

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1
You know the right answer?
What is the pH of a solution that results when 0.010mol HNO3 is added to 500.ml of a solution that i...
Questions


Mathematics, 16.04.2021 14:00

Chemistry, 16.04.2021 14:00



Mathematics, 16.04.2021 14:00

Mathematics, 16.04.2021 14:00

Social Studies, 16.04.2021 14:00

Mathematics, 16.04.2021 14:00

History, 16.04.2021 14:00

English, 16.04.2021 14:00


Chemistry, 16.04.2021 14:00

Mathematics, 16.04.2021 14:00


History, 16.04.2021 14:00

Mathematics, 16.04.2021 14:00

Business, 16.04.2021 14:00


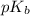 .
.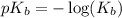
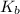 in this expression, we get:
in this expression, we get: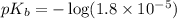
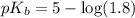
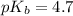
![pOH=pK_b+\log \frac{[Salt]}{[Base]}](/tpl/images/0522/5805/ac570.png)