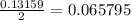Chemistry, 26.02.2020 19:29 whitneyt3218
For the reaction Ti(s)+2F2(g)→TiF4(s) compute the theoretical yield of the product (in grams) for each of the following initial amounts of reactants. Part A 5.0 g Ti, 5.0 g F2 Express your answer using two significant figures.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 03:00
Flourine is found to undergo 10% radioactivity decay in 366 minutes determine its halflife
Answers: 3

Chemistry, 22.06.2019 07:00
6what is the importance of water on earth? a) it keeps the top layer of the geosphere cool b) it allows life to exist c) it provides ice at the poles d) it creates earth's blue color from space
Answers: 2

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 22.06.2019 22:30
What is the work done by the electric force to move a 1 c charge from a to b?
Answers: 2
You know the right answer?
For the reaction Ti(s)+2F2(g)→TiF4(s) compute the theoretical yield of the product (in grams) for ea...
Questions




Computers and Technology, 19.03.2020 20:53

Chemistry, 19.03.2020 20:53


SAT, 19.03.2020 20:53

Mathematics, 19.03.2020 20:53




History, 19.03.2020 20:54

Mathematics, 19.03.2020 20:54


History, 19.03.2020 20:54

Mathematics, 19.03.2020 20:54

Mathematics, 19.03.2020 20:54



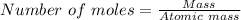
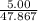
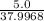
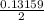 mole of Ti will react with the 0.13159 mole of F₂
mole of Ti will react with the 0.13159 mole of F₂