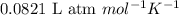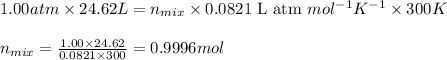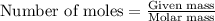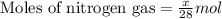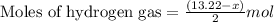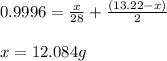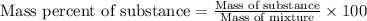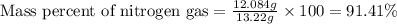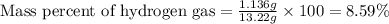Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:50
Acompound contains c, h, and o atoms. when 1.130 g of the compound is burned in oxygen, 1.064 g co2 and 0.3631 g h2o are produced. what is the empirical formula of this compound?
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 1

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1
You know the right answer?
Mixture of N 2 And H2 Gases weighs 13.22 g and occupies a volume of 24.62 L at 300 K and 1.00 atm. C...
Questions

History, 28.08.2019 05:30



Mathematics, 28.08.2019 05:30


Mathematics, 28.08.2019 05:30

Mathematics, 28.08.2019 05:30



Mathematics, 28.08.2019 05:30

History, 28.08.2019 05:30




Mathematics, 28.08.2019 05:30