Rank the following by their miscibility in
C10H22 (decane), from least miscible to
most:...

Rank the following by their miscibility in
C10H22 (decane), from least miscible to
most: H2O (water), C9H20 (nonane), CH3OH
(methanol), CH3CH2OH (ethanol).
1. CH3OH < CH3CH2OH < C9H20 < H2O
2. H2O < CH3OH < CH3CH2OH < C9H20
3. H2O < CH3CH2OH < CH3OH < C9H20
4. C9H20 < H2O < CH3OH < CH3CH2OH
5. CH3OH < C9H20 < CH3CH2OH < H2O

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:00
Different isotopes indicate that an element will have different numbers of
Answers: 2

Chemistry, 21.06.2019 21:00
Which of the following compounds does not contain molecules? question 2 options: co2 h2 nacl h2o
Answers: 1

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

You know the right answer?
Questions

Advanced Placement (AP), 19.03.2020 06:18



Spanish, 19.03.2020 06:18






Biology, 19.03.2020 06:18




Mathematics, 19.03.2020 06:19


History, 19.03.2020 06:19




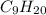 is a non-polar molecule so it is most soluble in decane molecule. Methanol and ethanol are the derivatives of hydrocarbon so they have more solubility than water but less solubility than nonane molecules.
is a non-polar molecule so it is most soluble in decane molecule. Methanol and ethanol are the derivatives of hydrocarbon so they have more solubility than water but less solubility than nonane molecules.


