
Chemistry, 28.02.2020 19:25 kayleegeise
The equilibrium constant for the reaction below, at a given temperature is 45.6. If the equilibrium concentrations of F2 and BrF3 are 1.24 x 10-1 M and 1.99 x 10-1 M respectively, calculate the equilibrium concentration of Br2. (4)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 23.06.2019 07:00
Select the correct answer. why are scientific models important in the study of science? a. they always involve critical mathematical calculations. b. they scientists understand complex ideas and objects that aren’t easy to handle. c. they enable scientists to popularize their work in society. d. they are required when conducting any peer review process. e. they are necessary for turning a hypothesis into a law.
Answers: 2

Chemistry, 23.06.2019 08:00
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 23.06.2019 11:50
Charles's law describes the relationship of the volume and temperature of gas at a constant mass and pressure. according to this law, what would happen to the temperature of the gas if its volume decreased from 1.0 l to 0.50 l?
Answers: 3
You know the right answer?
The equilibrium constant for the reaction below, at a given temperature is 45.6. If the equilibrium...
Questions

Computers and Technology, 19.10.2020 08:01

Computers and Technology, 19.10.2020 08:01

Chemistry, 19.10.2020 08:01


History, 19.10.2020 08:01

Geography, 19.10.2020 08:01

English, 19.10.2020 08:01

History, 19.10.2020 08:01


Advanced Placement (AP), 19.10.2020 08:01

Mathematics, 19.10.2020 08:01


English, 19.10.2020 08:01


Advanced Placement (AP), 19.10.2020 08:01


Mathematics, 19.10.2020 08:01

Chemistry, 19.10.2020 08:01


Mathematics, 19.10.2020 08:01

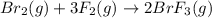
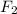 at equilibrium =
at equilibrium = 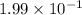
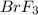 at equilibrium =
at equilibrium = 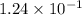
![K_c=\frac{[BrF_3]^2}{[Br_2][F_2]^3}](/tpl/images/0528/1843/a1098.png)
![45.6=\frac{(1.24\times 10^{-1})^2}{[Br_2]\times (1.99\times 10^{-1})^3}](/tpl/images/0528/1843/8cd8d.png)
![[Br_2]=0.0428M](/tpl/images/0528/1843/2a0dc.png)


