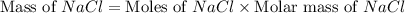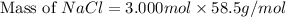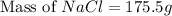Chemistry, 02.03.2020 18:22 squawk1738
The molality equation also requires the moles of solute. The molarity describes 3.000 moles of NaCl per 1 L of solution. Use the moles of NaCl to solve for the mass of NaCl. Note: use the molar mass of NaCl rounded to 4 significant figures.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
When curium-242 is bombarded with an alpha particle, two products are formed, one of which is a nudge on. what is the other product
Answers: 3

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2

Chemistry, 22.06.2019 19:50
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2

Chemistry, 23.06.2019 10:20
An engineer wishes to design a container that will hold 12.0 mol of ethane at a pressure no greater than 5.00x10*2 kpa and a temperature of 52.0 degrees celsius. what is the minimum volume the container can have?
Answers: 1
You know the right answer?
The molality equation also requires the moles of solute. The molarity describes 3.000 moles of NaCl...
Questions

World Languages, 16.04.2021 05:00

Mathematics, 16.04.2021 05:00




Mathematics, 16.04.2021 05:00

Mathematics, 16.04.2021 05:00

Mathematics, 16.04.2021 05:00

Mathematics, 16.04.2021 05:00


History, 16.04.2021 05:00


History, 16.04.2021 05:00

Mathematics, 16.04.2021 05:00





Mathematics, 16.04.2021 05:00

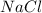 = 3.000 mol
= 3.000 mol