
Chemistry, 02.03.2020 23:32 naimareiad
A 0.10 mol sample of each of the four species in the reaction represented above is injected into a rigid, previously evacuated 1.0 L container. Which of the following species will have the highest concentration when the system reaches equilibrium? A) H2S(g) B) CH4(8) C) CS2(8) D) H2(8)

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 05:30
What royal scientist used the 29th day of frozen vapor to encounter elements for mastering new culinary creations?
Answers: 1

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 12:30
Which statement is true about this reaction? 14n+1h 15o it is a practical source of energy on earth. it occurs only outside the solar system. its product is heavier than each of its reactants. it shows the critical mass of an element.
Answers: 2
You know the right answer?
A 0.10 mol sample of each of the four species in the reaction represented above is injected into a r...
Questions

Chemistry, 27.11.2021 16:30


English, 27.11.2021 16:30



Mathematics, 27.11.2021 16:30

Social Studies, 27.11.2021 16:30





History, 27.11.2021 16:30

Computers and Technology, 27.11.2021 16:40

English, 27.11.2021 16:40

History, 27.11.2021 16:40

History, 27.11.2021 16:40


Mathematics, 27.11.2021 16:40

English, 27.11.2021 16:40

Mathematics, 27.11.2021 16:40

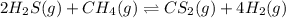
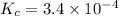
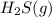 B)
B) 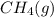 C)
C) 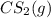 D)
D) 
![[H_2S]=[CH_4]=[CS_2]=[H_2]=\frac{0.10 mol}{1 L}=0.10 M](/tpl/images/0531/0970/4fb96.png)
![Q=\frac{[CS_2][H_2]^4}{[H_2S]^2[CH_4]}](/tpl/images/0531/0970/a102f.png)
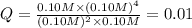
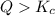
![K-c=\frac{[CS_2][H_2]^4}{[H_2S]^2[CH_4]}](/tpl/images/0531/0970/6df8e.png)
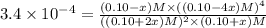
![[H_2S]=(0.10+2x) M=(0.10+2\times 0.099) M=0.298 M](/tpl/images/0531/0970/289d9.png)
![[CH_4]=(0.10+x) M=(0.10+ 0.099) M=0.199 M](/tpl/images/0531/0970/c1d03.png)
![[CS_2]=(0.10-x) M=(0.10- 0.099) M=0.001 M](/tpl/images/0531/0970/b47dc.png)
![[CH_4]=(0.10-4x) M=(0.10-4\times 0.099) M=-0.296 M](/tpl/images/0531/0970/11219.png)


