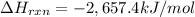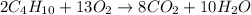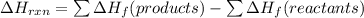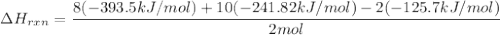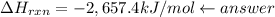Butane C4 H10 (g),(Delta. Hf = –125.7), combusts in the presence of oxygen to form CO2 (g) (Delta. Hf = –393.5 kJ/mol), and H2 O(g) (Delta. Hf = –241.82) in the reaction:
2 upper C subscript 4 upper H subscript 10 (g) plus 13 upper O subscript 2 (g) right arrow 8 upper C upper O subscript 2 plus 10 upper H subscript 2 upper O (g).
What is the enthalpy of combustion, per mole, of butane?
Use Delta H r x n equals the sum of delta H f of all the products minus the sum of delta H f of all the reactants..
–5,314.8 kJ/mol
–2,657.4 kJ/mol
2,657.4 kJ/mol
5,314.8 kJ/mol

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:00
In a water molecule, hydrogen and oxygen are held together by a(an) bond. a) double covalent b) ionic c) nonpolar covalent d) hydrogen e) polar covalent
Answers: 1

Chemistry, 23.06.2019 00:10
Apropane torch is lit inside a hot air balloon during preflight preparations to inflate the balloon. which condition of the gas remains constant
Answers: 2

Chemistry, 23.06.2019 06:30
What happens to the glucose molecule during the process of cellular respiration? (5 points) select one: a. it gets broken down. b. it forms oxygen. c. it builds muscles. d. it uses up energy.
Answers: 3

Chemistry, 23.06.2019 10:30
Me soon im confused much mass would a mole of hydrogen molecules contain? recall that hydrogen is diatomic. g/mol
Answers: 1
You know the right answer?
Butane C4 H10 (g),(Delta. Hf = –125.7), combusts in the presence of oxygen to form CO2 (g) (Delta. H...
Questions

Mathematics, 03.02.2021 16:00


English, 03.02.2021 16:00





Mathematics, 03.02.2021 16:00

English, 03.02.2021 16:00


Arts, 03.02.2021 16:00

Mathematics, 03.02.2021 16:00



Arts, 03.02.2021 16:00


English, 03.02.2021 16:00


Physics, 03.02.2021 16:00