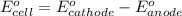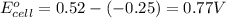A galvanic (voltaic) cell consists of an electrode composed of nickel in a 1.0 M nickel(II) ion solution and another electrode composed of copper in a 1.0 M copper(I) ion solution, connected by a salt bridge. Calculate the standard potential for this cell at 25 °C .

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2


Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3

Chemistry, 22.06.2019 23:30
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3
You know the right answer?
A galvanic (voltaic) cell consists of an electrode composed of nickel in a 1.0 M nickel(II) ion solu...
Questions


Mathematics, 20.04.2021 17:20


Mathematics, 20.04.2021 17:20



Mathematics, 20.04.2021 17:20

Mathematics, 20.04.2021 17:20

Mathematics, 20.04.2021 17:20







Mathematics, 20.04.2021 17:20


History, 20.04.2021 17:20


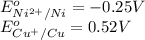
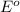 reduction potential will always get reduced and will undergo reduction reaction.
reduction potential will always get reduced and will undergo reduction reaction.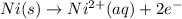
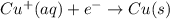 ( × 2)
( × 2)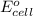 of the reaction, we use the equation:
of the reaction, we use the equation: