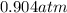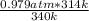Chemistry, 06.03.2020 23:49 RoyalGurl01
A sealed container holding 0.0255 L of an ideal gas at 0.979 atm and 67 ∘ C is placed into a refrigerator and cooled to 41 ∘ C with no change in volume. Calculate the final pressure of the gas. P = atm

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 22:00
All of the following are homogeneous mixtures except a) sugar dissolved in water. b) orange juice. c) coffee with cream. d) household vinegar. e) apple juice
Answers: 1

Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2

Chemistry, 23.06.2019 03:30
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1

Chemistry, 23.06.2019 15:00
What is the volume in liters of 7500 g of helium atoms. assume stp conditions.
Answers: 1
You know the right answer?
A sealed container holding 0.0255 L of an ideal gas at 0.979 atm and 67 ∘ C is placed into a refrige...
Questions

Social Studies, 26.06.2019 06:00

English, 26.06.2019 06:00


Mathematics, 26.06.2019 06:00

Geography, 26.06.2019 06:00

Physics, 26.06.2019 06:00

English, 26.06.2019 06:00

History, 26.06.2019 06:00

Mathematics, 26.06.2019 06:00

Computers and Technology, 26.06.2019 06:00


Biology, 26.06.2019 06:00



Mathematics, 26.06.2019 06:00

History, 26.06.2019 06:00



English, 26.06.2019 06:00

History, 26.06.2019 06:00