

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1



Chemistry, 23.06.2019 06:30
Generally, observed behavior that can be formulated into a statement, sometimes mathematical in nature, is called a(n): a. observation. b. measurement. c. theory. d. natural law. e. experiment.
Answers: 2
You know the right answer?
A weak acid is titrated with 0.1236 M NaOH. From the titration curve you determine that the equivale...
Questions





Mathematics, 04.03.2021 02:40

Mathematics, 04.03.2021 02:40

Computers and Technology, 04.03.2021 02:40

Mathematics, 04.03.2021 02:40

Mathematics, 04.03.2021 02:40

Mathematics, 04.03.2021 02:40


Biology, 04.03.2021 02:40


Mathematics, 04.03.2021 02:40


Geography, 04.03.2021 02:40


History, 04.03.2021 02:40


Mathematics, 04.03.2021 02:40

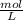 × 12.43 mL
× 12.43 mL

