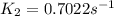Chemistry, 07.03.2020 04:09 beelcypher
The rate constant of a first-order reaction is 3.46 × 10−2 s−1 at 298 K. What is the rate constant at 350 K if the activation energy for the reaction is 50.2 kJ/mol

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
The rate constant of a first-order reaction is 3.46 × 10−2 s−1 at 298 K. What is the rate constant a...
Questions


Advanced Placement (AP), 15.12.2020 05:10






Mathematics, 15.12.2020 05:20

Medicine, 15.12.2020 05:20

Mathematics, 15.12.2020 05:20






English, 15.12.2020 05:20

Physics, 15.12.2020 05:20

Mathematics, 15.12.2020 05:20

History, 15.12.2020 05:20

Mathematics, 15.12.2020 05:20

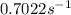 is the rate constant at 350 K if the activation energy for the reaction is 50.2 kJ/mol.
is the rate constant at 350 K if the activation energy for the reaction is 50.2 kJ/mol.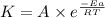
![\log (\frac{K_2}{K_1})=\frac{Ea}{2.303\times R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0537/5727/6d953.png)
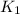 = rate constant at 298 K=
= rate constant at 298 K= 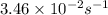
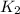 = rate constant at 350 K =?
= rate constant at 350 K =?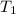 = initial temperature = 298 K
= initial temperature = 298 K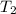 = final temperature = 350 K
= final temperature = 350 K![\log (\frac{K_2}{3.46\times 10^{-2} s^{-1}})=\frac{50200 J/mol}{2.303\times 8.314J/mole.K}[\frac{1}{350 K}-\frac{1}{298 K}]](/tpl/images/0537/5727/978f6.png)