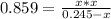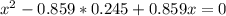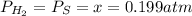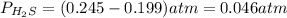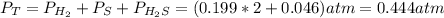Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 10:00
A50.0g sample of liquid water at 0.0 c ends up as ice at -20.0 c. how much energy is involved in this change?
Answers: 1

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1
You know the right answer?
At a certain temperature, the K p for the decomposition of H 2 S is 0.859 . H 2 S ( g ) − ⇀ ↽ − H 2...
Questions


Mathematics, 09.11.2020 22:40



English, 09.11.2020 22:40

Mathematics, 09.11.2020 22:40


Social Studies, 09.11.2020 22:40


Chemistry, 09.11.2020 22:40




Mathematics, 09.11.2020 22:40


Spanish, 09.11.2020 22:40


History, 09.11.2020 22:40


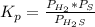 (1)
(1)