

Answers: 2
Another question on Chemistry



Chemistry, 23.06.2019 15:50
Astable atom that has a large nucleus most likely contains 1. more neutrons than protons. 2.more protons than neutrons. 3.equal numbers of protons and neutrons. 4.changing numbers of protons and neutrons.
Answers: 1

Chemistry, 23.06.2019 21:30
25 points, the sap from a maple tree is an example of a concentrated solution weak solvent dilute solution suspension
Answers: 1
You know the right answer?
Consider the following reaction, equilibrium concentrations, and equilibrium constant at a particula...
Questions


Mathematics, 23.06.2021 01:50

SAT, 23.06.2021 01:50


Mathematics, 23.06.2021 01:50



Mathematics, 23.06.2021 01:50






English, 23.06.2021 01:50


History, 23.06.2021 01:50


Mathematics, 23.06.2021 01:50

Biology, 23.06.2021 01:50

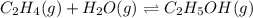
![[C_2H_4]=0.015M](/tpl/images/0537/7510/e5922.png)
![[H_2O]=?](/tpl/images/0537/7510/d3a13.png)
![[C_2H_5OH]=1.69 M](/tpl/images/0537/7510/ae95d.png)
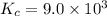
![K_c=\frac{[C_2H_5OH]}{[C_2H_4][H_2O]}](/tpl/images/0537/7510/88e9b.png)
![9.0\times 10^3=\frac{1.69 M}{0.015 M\times [H_2O]}](/tpl/images/0537/7510/9ae4d.png)
![[H_2O]=\frac{1.69 M}{0.015 M\times 9\timers 10^3}=0.0125 M](/tpl/images/0537/7510/e2bbe.png)



