
Chemistry, 07.03.2020 05:36 damien1030
Calculate the pressures of NO, Cl2, and NOCl in an equilibrium mixture produced by the reaction of a starting mixture with 8.2 atm NO and 4.1 atm Cl2. (Hint: Kp is relatively large; assume the reaction goes to completion then comes back to equilibrium.)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

You know the right answer?
Calculate the pressures of NO, Cl2, and NOCl in an equilibrium mixture produced by the reaction of a...
Questions




Mathematics, 27.11.2019 19:31


Mathematics, 27.11.2019 19:31



Mathematics, 27.11.2019 19:31

Mathematics, 27.11.2019 19:31









English, 27.11.2019 19:31

 , and NOCl in an equilibrium mixture produced by the reaction of a starting mixture with 8.2 atm NO and 4.1 atm
, and NOCl in an equilibrium mixture produced by the reaction of a starting mixture with 8.2 atm NO and 4.1 atm 
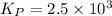
 of the reaction is as follows.
of the reaction is as follows.![K_{P} = \frac{[NOCl]^{2}}{[NO]^{2}[Cl_{2}]}](/tpl/images/0537/9927/829f4.png)

 = 3.8
= 3.8

