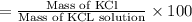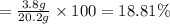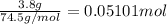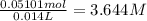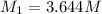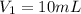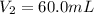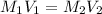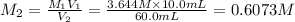Chemistry, 10.03.2020 03:19 jbrown76241
In a laboratory experiment, a 14.0 mL sample of KCl solution is poured into an evaporating dish with a mass of 24.10 g. The combined mass of the evaporating dish and KCl solution is 44.30 g. After heating, the evaporating dish and dry KCl have a combined mass of 27.90 g.
(a) What is the mass percent (m/m) of the KCl solution?
(b) What is the molarity ( M) of the KCl solution?
(c) If water is added to 10.0 mL of the initial KCl solution to give a final volume of 60.0 mL, what is the molarity of the diluted KCl solution?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 23.06.2019 01:20
How can parts of a solution be separated by chromatography?
Answers: 1

Chemistry, 23.06.2019 01:30
At a certain temperature the rate of this reaction is first order in hi with a rate constant of : 0.0632s2hig=h2g+i2g suppose a vessel contains hi at a concentration of 1.28m . calculate how long it takes for the concentration of hi to decrease to 17.0% of its initial value. you may assume no other reaction is important. round your answer to 2 significant digits.
Answers: 1
You know the right answer?
In a laboratory experiment, a 14.0 mL sample of KCl solution is poured into an evaporating dish with...
Questions


Biology, 26.07.2019 20:30


English, 26.07.2019 20:30

Mathematics, 26.07.2019 20:30


Mathematics, 26.07.2019 20:30



Biology, 26.07.2019 20:30



Mathematics, 26.07.2019 20:30




Social Studies, 26.07.2019 20:30



Mathematics, 26.07.2019 20:30