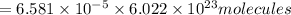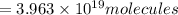Hydrogen gas, H2, reacts with nitrogen gas, N2, to form ammonia gas, NH3, according to the equation
3H2(g)+N2(g)→2NH3(g)
NOTE: Throughout this tutorial use molar masses expressed to five significant figures.
Part A
How many moles of NH3 can be produced from 18.0 mol of H2 and excess N2?
Part B
How many grams of NH3 can be produced from 4.50 mol of N2 and excess H2.
Part C
How many grams of H2 are needed to produce 12.57 g of NH3?
Part D
How many molecules (not moles) of NH3 are produced from 1.99×10−4 g of H2?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:40
Which statement can best be concluded from the ideal gas law?
Answers: 2

Chemistry, 22.06.2019 12:20
Adeuteron, 21h, is the nucleus of a hydrogen isotope and consists of one proton and one neutron. the plasma of deuterons in a nuclear fusion reactor must be heated to about 3.02×108 k . what is the rms speed of the deuterons? express your answer using two significant figures.
Answers: 1

Chemistry, 22.06.2019 16:00
About 3% of the water on earth is freshest. only about 40% of that freshwater is available for human use. why is so much freshwater unavailable for human use?
Answers: 2

Chemistry, 22.06.2019 23:00
Which of your 24 wells had indications that a chemical reaction occurred? how were you able to tell that a chemical reaction occurred? which of your 24 wells had indications that a physical reaction occurred? how were you able to tell that a physical reaction occurred? report on both mixing and evaporation. make a general statement about whether your hypotheses were validated or rejected. must your hypotheses be correct for this to be a successful laboratory?
Answers: 3
You know the right answer?
Hydrogen gas, H2, reacts with nitrogen gas, N2, to form ammonia gas, NH3, according to the equation<...
Questions

Geography, 04.04.2020 20:21




Mathematics, 04.04.2020 20:21

Social Studies, 04.04.2020 20:21

Medicine, 04.04.2020 20:21



Chemistry, 04.04.2020 20:21


History, 04.04.2020 20:21



Chemistry, 04.04.2020 20:21

Social Studies, 04.04.2020 20:21


Mathematics, 04.04.2020 20:21

Chemistry, 04.04.2020 20:21

History, 04.04.2020 20:21

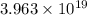 molecules of ammonia will be produced.
molecules of ammonia will be produced.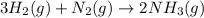
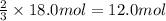 of ammonia
of ammonia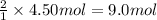 of ammonia
of ammonia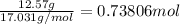
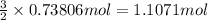 of hydrogen gas
of hydrogen gas 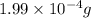
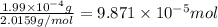
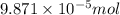 moles of hydrogen gas will give ;
moles of hydrogen gas will give ;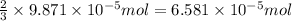 of ammonia.
of ammonia.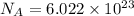 molecules/ atoms
molecules/ atoms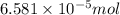 .
.