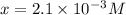Chemistry, 10.03.2020 08:25 ballin2126
The acid-dissociation constant for benzoic acid (C6H5COOH) is 6.3×10−5. Calculate the equilibrium concentration of H3O+ in the solution if the initial concentration of C6H5COOH is 7.0×10−2 M .

Answers: 1
Another question on Chemistry


Chemistry, 23.06.2019 06:20
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1

Chemistry, 23.06.2019 15:20
Plzzz ? which stores information in discrete steps? a magnet and coil of wire compact discs plastic records amplified speakers
Answers: 2

Chemistry, 23.06.2019 16:00
Instructions: the table below explains the average rate at which some geologic processes occur. calculate the amount of sea level change, erosion, and uplift for 100,000 years, 1,000,000 years, and 10,000,000 years. remember, 100 cm = 1 m. fill out the table completely and answer the below questions in complete sentences. for with completing the table, use this video: if you need directions on how to submit your assignment, click on the link due by sunday at midnight for full credit. if submitted late, you will receive a 30% grade deduction. check your pacing guide for assignment dates. process rate per 1,000 years after 100,000 years after 1,000,000 years after 10,000,000 years sea level changes 10 m 1000 10000 100000 regional erosion 2 m 200 2000 20000 uplift 10 cm 1. what is the fastest process, sea level changes, erosion, or uplift? 2. what is the slowest process, seal level changes, erosion, or uplift?
Answers: 3
You know the right answer?
The acid-dissociation constant for benzoic acid (C6H5COOH) is 6.3×10−5. Calculate the equilibrium co...
Questions






Mathematics, 27.02.2020 05:23



Mathematics, 27.02.2020 05:23

Biology, 27.02.2020 05:23










History, 27.02.2020 05:23

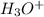 in the solution is,
in the solution is, 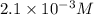
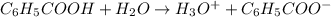
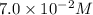
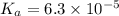
![K_a=\frac{[H_3O^+][C_6H_5COO^-]}{[C_6H_5COOH]}](/tpl/images/0540/6257/de276.png)
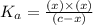
![6.3\times 10^{-5}=\frac{(x)\times (x)}{[(7.0\times 10^{-2})-x]}](/tpl/images/0540/6257/1bf1d.png)