
Chemistry, 10.03.2020 17:34 stormmesa1
An element P combines with another element Q in the weight ratio 1: 5. If 15 g of P is made to react with 62g of Q to a compound. What will be the total weight of the new compound formed?
(A) 60 g
(B) 77 g
(C) 74.4 g
(D) 74 g
(E) 66 g

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 23.06.2019 13:20
What volume of 24% trichloroacetic acid (tca) is needed to prepare eight 3 ounce bottles of 10% tca solution?
Answers: 2

Chemistry, 23.06.2019 18:00
Astudent measured the ph for four solutions which ph indicated the lowest hydronium ion or concentration
Answers: 1

Chemistry, 23.06.2019 18:10
Which property of a substance can be determined using a ph indicat
Answers: 3
You know the right answer?
An element P combines with another element Q in the weight ratio 1: 5. If 15 g of P is made to react...
Questions


History, 01.02.2020 07:44


Business, 01.02.2020 07:44

Mathematics, 01.02.2020 07:44

Chemistry, 01.02.2020 07:44

Mathematics, 01.02.2020 07:44

Mathematics, 01.02.2020 07:44

Mathematics, 01.02.2020 07:44

Mathematics, 01.02.2020 07:44



Mathematics, 01.02.2020 07:44

History, 01.02.2020 07:44

History, 01.02.2020 07:44



Social Studies, 01.02.2020 07:44


History, 01.02.2020 07:44

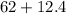 grams which will give us with 74.4 grams of compound.
grams which will give us with 74.4 grams of compound. 

