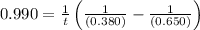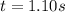Chemistry, 11.03.2020 06:13 dianacastro8298
The rate constant for this second‑order reaction is 0.990 M − 1 ⋅ s − 1 0.990 M−1⋅s−1 at 300 ∘ C. 300 ∘C. A ⟶ products A⟶products How long, in seconds, would it take for the concentration of A A to decrease from 0.650 M 0.650 M to 0.380 M?

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1
You know the right answer?
The rate constant for this second‑order reaction is 0.990 M − 1 ⋅ s − 1 0.990 M−1⋅s−1 at 300 ∘ C. 30...
Questions

Mathematics, 10.12.2020 20:00



Mathematics, 10.12.2020 20:00

History, 10.12.2020 20:00



History, 10.12.2020 20:00


Mathematics, 10.12.2020 20:00








History, 10.12.2020 20:00


Mathematics, 10.12.2020 20:00

![k=\frac{1}{t}\left (\frac{1}{[A]}-\frac{1}{[A]_o}\right)](/tpl/images/0542/6727/5ea71.png)
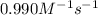
![[A]_o](/tpl/images/0542/6727/9caf5.png) = Initial concentration = 0.650 M
= Initial concentration = 0.650 M