
Chemistry, 11.03.2020 17:49 tylerkitchen44
What volume (in L) of oxygen will be required to produce 77.4 L of water vapor in the reaction below?
2c2H6(g)+7O2(g)--->4CO2(g)+6H2O( g)
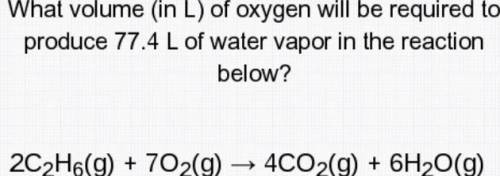

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2
You know the right answer?
What volume (in L) of oxygen will be required to produce 77.4 L of water vapor in the reaction below...
Questions






World Languages, 14.03.2022 21:40




History, 14.03.2022 21:40

English, 14.03.2022 21:40

Chemistry, 14.03.2022 21:40





Mathematics, 14.03.2022 21:50


Social Studies, 14.03.2022 21:50



