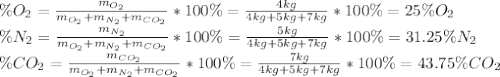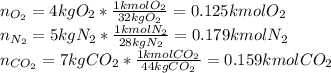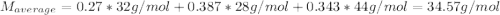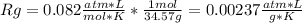Chemistry, 12.03.2020 00:29 damiangibson2
A gas mixture consists of 4 kg of O2, 5 kg of N2, and 7 kg of CO2. Determine (a) the mass fraction of each component, (b) the mole fraction of each component, and (c) the average molar mass and gas constant of the mixture.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 18:10
Which statements describe polyatomic ions? check all that apply. polyatomic ions have many charges. polyatomic ions have one overall charge. polyatomic ions repel other ions to form ionic bonds. polyatomic ions attract other ions to form ionic bonds. polyatomic ions are made up of only one type of atom. polyatomic ions are made up of two or more types of atoms.
Answers: 2

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3
You know the right answer?
A gas mixture consists of 4 kg of O2, 5 kg of N2, and 7 kg of CO2. Determine (a) the mass fraction o...
Questions

Mathematics, 15.02.2020 16:25

Chemistry, 15.02.2020 16:26


Chemistry, 15.02.2020 16:34

English, 15.02.2020 16:37



Mathematics, 15.02.2020 16:44


Health, 15.02.2020 16:45

Health, 15.02.2020 16:47

Health, 15.02.2020 16:48

Mathematics, 15.02.2020 17:01



Mathematics, 15.02.2020 17:04


Chemistry, 15.02.2020 17:05


Mathematics, 15.02.2020 17:07